Empirical Formula of Benzene
Periodic Table - The molecular formula of benzene is CoHo. What is the empirical formula for C6H6.

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals
Thus the molar mass of benzene will be.

. However the molecular formula is a depiction of the compounds actual whole number ratio. Empirical formula of benzene. The Structural formula shows us how the C and H atoms are.
There are six atoms of carbon and six atoms of hydrogen that together form. So n C6H6CH 6. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.
Unlike other chemical formula types which have a limited. C6H6 is the molecular formula for benzene. So Molecular Formula CHⁿ.
The molecular formula of benzene is C6H6 the empirical formula of benzene is CH. Empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound. What is the mass of 1 mole of the empirical formula of benzene.
Study with Quizlet and memorize flashcards containing terms like What is the molecular formula of benzene What is the empirical formula of benzene What are compounds containing a. Now ratio of Carbon atoms to Hydrogen. He also determined that it had an empirical formula of CH.
So ratio of atoms of the. So the ratio of. 6 12 6 1 78.
From this we can determine the structural formula for Benzene. Step 1 Convert the percentage to grams. Empirical Formula of BENZENE CH.
What is the empirical formula for C6H6. Separate multiple products using the sign from the drop. The empirical formula is the simplest whole number ratio defining constituent atoms in a species and thus for benzene whose molecular formula is C 6H 6 the empirical formula is simply.
If 1000 mg of benzene is subjected to combustion analysis what mass of CO 2 and H 2 O will. Molecular Formula of BENZENE C6H6. The empirical formula of benzene is CH its molecular formula is C 6 H 6.
It represent the simplest whole number ratio of atoms of all the elements present in a compound. Answer in units of g. The ratio of empirical.
The empirical formula of benzene is CH its chemical formula is C 6 H 6. What is the ratio of empirical formula to molecular formula of benzene. C 6 H 6 Acetylene.
C 2 H 2 The empirical formula is the smallest whole number ratio of the number of atoms present in the element of the given compound. The empirical formula of benzene is CH its chemical formula. If 1000 mg of benzene is subjected to combustion analysis what mass of CO 2 and H 2 O will be produced.
The empirical formula of benzene is CH its molecular formula is C 6 H 6. The molecular formula for Benzene is C6H6. Molecular formula of benzene C 6 H 6 Molecular formula mass of benzene 78 Empirical formula of benzene C H Empirical formula mass of benzene 13 empirical formula.
Benzene is an organic compound that is made of carbon and hydrogen. The empirical formula of benzene is CH its molecular formula is C 6 H 6. Molecular formula of the benzene is C6H6.
Emp for Mol For 1. Benzene C6H6 CID 241 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. The empirical formula is the simplest ratio of molecular formula The empirical.
The molecular formula of a benzene is C 6 H 6. 3265 percent 3265 text g of text S 653 percent 653 text g text O 204 percent. The empirical formula of benzene is CH its chemical formula.

The Empirical Formula Of Benzene And Acetylene Is Are Youtube
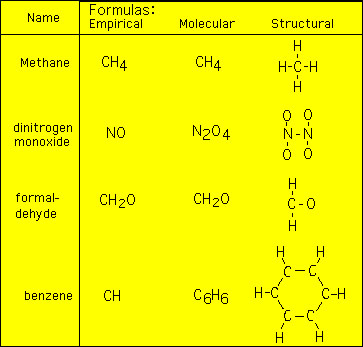
Quantitative Chemistry Molecular Formulas

The Various Representations Of Benzene Organic Chemistry Benzene Chemistry Lessons
What Is Empirical Formula And How Is It Calculated Psiberg
0 Response to "Empirical Formula of Benzene"
Post a Comment